ERIVANCE BCC: Efficacy outcomes
Pivotal study demonstrating clinically meaningful benefit of Erivedge® in advanced BCC1–3
Study Design
Phase II, international, single-arm, multicentre, two-cohort study1–3
This website is a global information resource. It is intended for healthcare professionals outside of the United States of America (US) who are interested in information on Erivedge®.
This website is not country-specific and therefore may contain information that is not applicable to your country. Please refer to your local Prescribing Information for full details.
If you are a US healthcare professional, click here
If you are a US patient, click here
All Countries
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Argentina
Local HCP Portal: https://www.dialogoroche.com/ar/home/sitios_de_productos/info_para_preescribir.html
Request Medical Information: https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Australia
http://www.roche-australia.com/
Local HCP Portal: https://www.rocheinteract.com.au/medicine-and-resources/Erivedge.html
Request Medical Information: https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Bulgaria
Local HCP Portal: https://www.rochemd.bg/bg_bg/products1/oncology/erivedge.protected.html
Request Medical Information: https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Croatia
Local HCP Portal: https://www.rochepro.hr/hr_hr/products/erivedge.protected.html
Request Medical Information: https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Czech Republic
Local HCP Portal: https://www.mojemedicina.cz/cs_cz/lecive-pripravky/onkologie/erivedge.protected.html
Request Medical Information: https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Finland
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
France
Local HCP Portal: https://professionnels.roche.fr/fr_fr/products/oncologie/erivedge01.protected.html
Request Medical Information: https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Germany
https://www.roche.de/index.html#Pharma
Request Medical Information: https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Greece
Local HCP Portal: https://www.rochemed.gr/el_gr/products/erivedge.protected.html
Request Medical Information: https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Hungary
Local HCP Portal: https://www.rochetudastar.hu/hu_hu/gyogyszereink/erivedge.protected.html
Request Medical Information: https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Israel
Local HCP Portal: https://www.roche4med.co.il/he_il/products/erivedge-vismodegib.html
Request Medical Information: https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Italy
Local HCP Portal: https://www.infomedics.it/it_it/products/erivedge.protected.html
Request Medical Information: https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Netherlands
Local HCP Portal: https://www.roche4professionals.nl/nl_nl/geneesmiddelen/oncologie-geneesmiddelen/Erivedge/Productinformatie.protected.html
Request Medical Information: https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Poland
Local HCP Portal: https://dlalekarzy.roche.pl/pl_pl/products/onkologia/erivedge.protected.html
Link to the local version of Educational Materials
Request Medical Information: https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Portugal
https://www.roche.pt/corporate/
Local HCP Portal: https://www.rochenet.pt/pt_pt/medicamentos-roche/site-erivedge.protected.html
Request Medical Information: https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Romania
Local HCP Portal: https://www.e-roche.ro/ro_ro/products/produsele-roche/erivedge.protected.html
Request Medical Information: https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Spain
Local HCP Portal: https://www.rocheplus.es/es_es/products/oncologia/erivedge.protected.html
Request Medical Information: https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Sweden
Local HCP Portal: https://www.rocheonline.se/sv_se/onkologi/lakemedel/erivedge.html
Request Medical Information: https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Switzerland
Request Medical Information:https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Turkey
https://www.medikaynak.com/u/erivedge
Request Medical Information:https://medinfo.roche.com/
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
United States of America
Local HCP Portal: https://www.erivedge.com/
Request Medical Information: https://www.gene.com/contact-us/submit-medical-inquiry
By choosing your country, you will be redirected to a Healthcare Professional Portal. Please be aware that it may require registration or log-in to access certain areas.
Study Design
Phase II, international, single-arm, multicentre, two-cohort study1–3
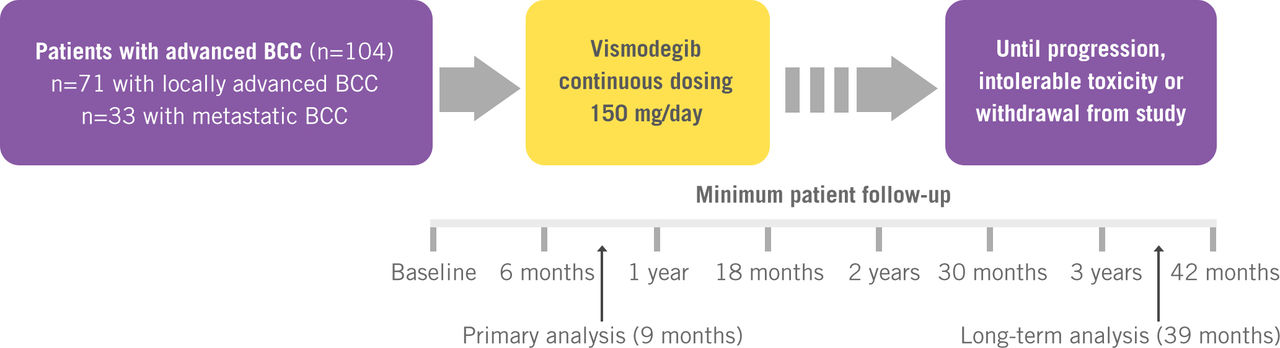
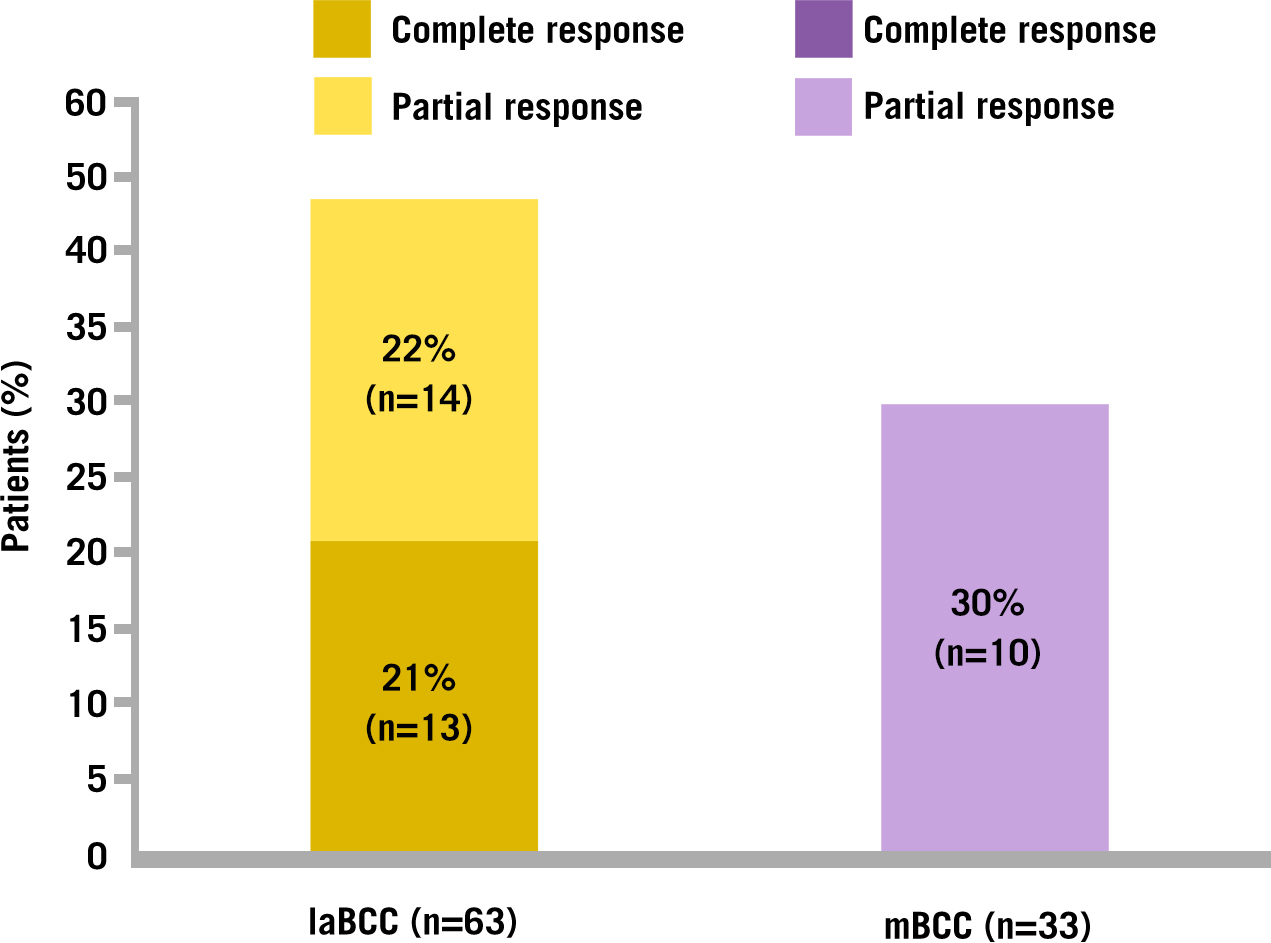
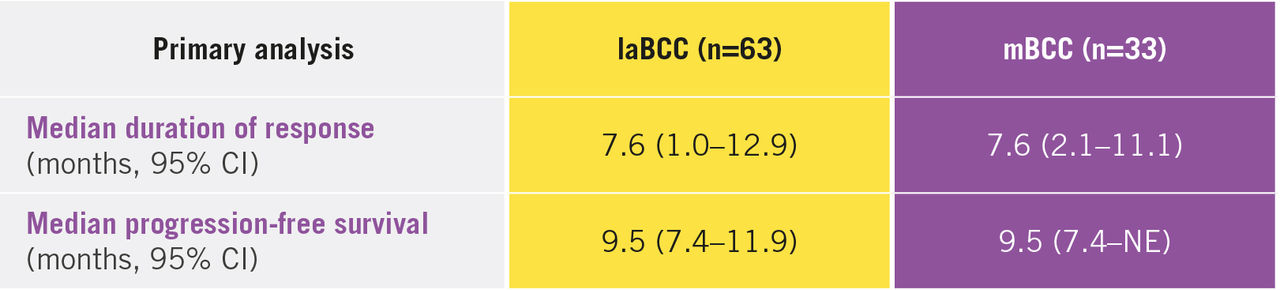
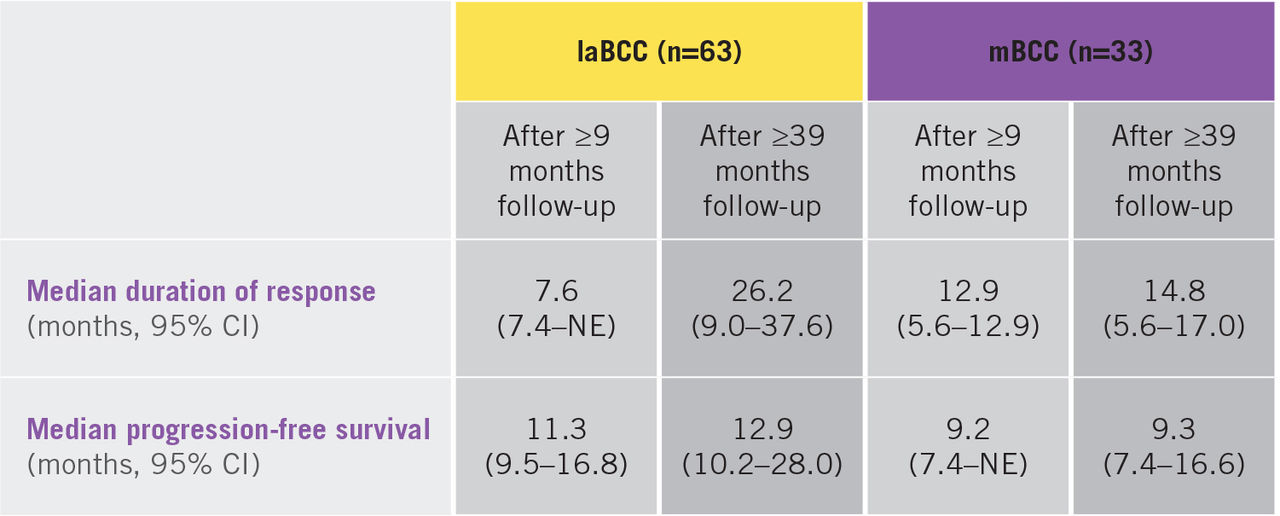
Erivedge® treatment has demonstrated clinically meaningful lesion reductions after 9 months, with a 43% and 30% objective response rate for patients with laBCC and mBCC, respectively.
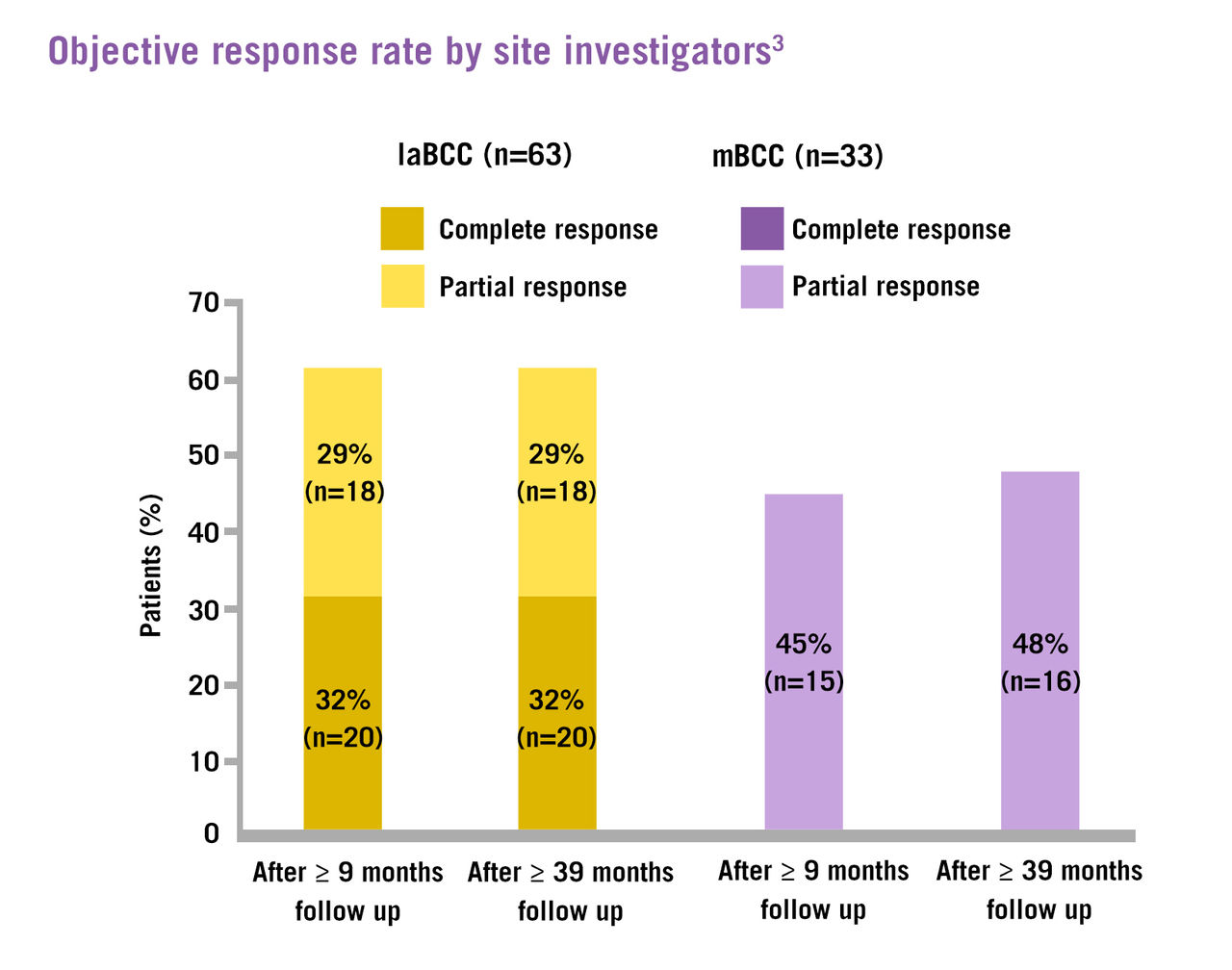
Erivedge® treatment has demonstrated clinically meaningful and lasting lesion reductions, with a 32% complete response rate and 49% objective response rate for patients with laBCC and mBCC, respectively
References
References:
1. Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of Hedgehog pathway inhibitors in Basal cell carcinoma. Mol Cancer Ther. 2015 Mar;14:633-41
2. eMC. Erivedge 150 mg hard capsules - Summary of Product Characteristics June 2022; available at: https://www.ema.europa.eu/documents/product-information/erivedge-epar-product-information_en.pdf.
Date of preparation with December 2024 I M-XX-00003514
